All Atoms Of An Element Have The Same
| Lecture Notes - Part 1 | Physical Structure |
But, all atoms are not the same. You know that the number of protons in an atom determines what element you have. For instance hydrogen has one proton, carbon has six. The difference in the number of protons and neutrons in atoms account for many of the different properties of elements.
- Atoms of two different elements, by the definition of an element, MUST have different atomic numbers. We define the element helium, for example, to be the atoms that have 2 protons, or atomic number (Z) = 2. Two atoms of different elements can certainly have the same mass number, though it is much more common in larger atoms.
- All atoms have at least one proton in their core, and the number of protons determines which kind of element an atom is. For example, an oxygen atom has 8 protons. If you were somehow able to change the proton number of this atom to 7, even if everything else remained the same, it would no longer be an oxygen atom, it would be nitrogen.
- All atoms of the same have the same properties Elements Compounds Mixtures Chemicals Get the answers you need, now! Averyandkelly averyandkelly.
Quick Check
Read each of the Quick Questions below and write down your answer.
When you have finished the test, click on the ANSWERS button and see how well you have done.
Test Number PS-1030
Quick Questions
1) All atoms are the same. (True / False)?
2) All atoms of the same element are the same. (True / False)?


3) All atoms contain neutrons. (True / False)?
4) Protons have more mass than neutrons. (True / False)?
5) Electrons have less mass than either protons or neutrons. (True / False)?
6) In a neutral atom there are always equal numbers of protons and electrons. (True / False)?
7) Isotopes are a family of atoms all of which have the same number of electrons. (True / False)?
8) Electrons are found in clouds called orbitals surrounding the atomic centers. (True / False)?
9) Electrons are sometimes exchanged between atoms. (True / False)?
10) Ions are atoms that have gained or lost electrons. (True / False)?
11) Ions always have a positive charge. App store on mac. (True / False)?
12) Atoms are at their most stable when their outermost electron energy level is completely filled or completely empty of electrons. (True / False)?
13) Hydrogen atoms and Helium atoms both have two protons in their atomic centers, but different numbers of neutrons. (True / False)?
14) Hydrogen and Deuterium atoms both have the same number of protons in their atomic centers, but different numbers of neutrons. (True / False)?
15) The atomic mass of all isotopes within a family of atoms is always the same. (True / False)?
16) The atomic number of all isotopes within a family of atoms is always the same. (True / False)?
17) Atoms of the same element can sometimes share two electrons. (True / False)?
18) Atoms of different elements can sometimes share electrons. (True / False)?
19) Atoms share electrons so as to fill energy levels. (True / False)?
20) Only eight electrons can occupy an one orbital. (True / False)?
21) Two electrons shared between two atoms creates a force or bond that holds the atoms together. (True / False)?
22) More than two electrons are sometimes shared between atoms. (True / False)?
23) Covalent bonds can only form between atoms that can ionize. (True / False)?
24) Ionization of a hydrogen atom produces a proton. (True / False)?
25) Methane is an isotope of carbon. Autocad for mac 2019 torrent. (True / False)?
26) Water molecules are smaller than molecules of oxygen gas. (True / False)?
Parts Of An Atom
27) Hydrogen and oxygen share the electrons equally in the covalent bonds of a water molecule. (True / False)?
28) A polar molecule is one that is most stable at the poles of reaction. (True / False)?
29) Water is a liquid at room temperature because the water molecules are strongly sympathetic to one another. (True / False)?
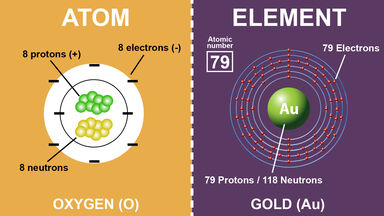
All Atoms Of An Element Have The Same Atomic Number
30) Hydrophilic substances dissolve readily and easily in water. (True / False)?
All Atoms Of An Element Have The Same Number
| Answers to Part 1 | Lecture Notes - Part 1 |
| Physical Structure | |
 Science at a Distance
Science at a Distance © 1997, 1998, 1999, 2000, Professor John Blamire
